MONITORING, SURVEILLANCE AND NATIONAL PLANS
AMR CONTROL 2015 47
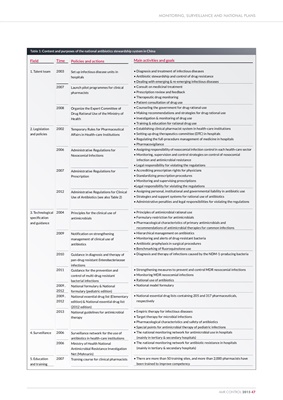
Table 1: Content and purposes of the national antibiotics stewardship system in China
Field
1. Talent team
2. Legislation
and policies
3. Technological
specification
and guidance
4. Surveillance
5. Education
and training
Time
2003
2007
2008
2002
2006
2007
2012
2004
2009
2010
2011
2009,
2012
2009,
2012
2013
2006
2006
2007
Policies and actions
Set up infectious disease units in
hospitals
Launch pilot programmes for clinical
pharmacists
Organize the Expert Committee of
Drug Rational Use of the Ministry of
Health
Temporary Rules for Pharmaceutical
Affairs in Health-care Institutions
Administrative Regulations for
Nosocomial Infections
Administrative Regulations for
Prescription
Administrative Regulations for Clinical
Use of Antibiotics (see also Table 2)
Principles for the clinical use of
antimicrobials
Notification on strengthening
management of clinical use of
antibiotics
Guidance in diagnosis and therapy of
pan-drug resistant Enterobacteriaceae
infections
Guidance for the prevention and
control of multi-drug resistant
bacterial infections
National formulary & National
formulary (pediatric edition)
National essential drug list (Elementary
edition) & National essential drug list
(2012 edition)
National guidelines for antimicrobial
therapy
Surveillance network for the use of
antibiotics in health-care institutions
Ministry of Health National
Antimicrobial Resistance Investigation
Net (Mohnarin)
Training course for clinical pharmacists
Main activities and goals
• Diagnosis and treatment of infectious diseases
• Antibiotic stewardship and control of drug resistance
• Dealing with emerging & re-emerging infectious diseases
• Consult on medicinal treatment
• Prescription review and feedback
• Therapeutic drug monitoring
• Patient consultation of drug use
• Counseling the government for drug rational use
• Making recommendations and strategies for drug rational use
• Investigation & monitoring of drug use
• Training & education for rational drug use
• Establishing clinical pharmacist system in health-care institutions
• Setting up drug therapeutics committee (DTC) in hospitals
• Regulating the full-procedure management of medicine in hospitals
• Pharmacovigilance
• Assigning responsibility of nosocomial infection control in each health-care sector
• Monitoring, supervision and control strategies on control of nosocomial
infection and antimicrobial resistance
• Legal responsibility for violating the regulations
• Accrediting prescription rights for physicians
• Standardizing prescription procedures
• Monitoring and supervising prescriptions
•Legal responsibility for violating the regulations
• Assigning personal, institutional and governmental liability in antibiotic use
• Strategies and support systems for rational use of antibiotics
• Administrative penalties and legal responsibilities for violating the regulations
• Principles of antimicrobial rational use
• Formulary restriction for antimicrobials
• Pharmacological characteristics of primary antimicrobials and
recommendations of antimicrobial therapies for common infections
• Hierarchical management on antibiotics
• Monitoring and alerts of drug-resistant bacteria
• Antibiotic prophylaxis in surgical procedures
• Benchmarking of fluoroquinolone use
• Diagnosis and therapy of infections caused by the NDM-1-producing bacteria
• Strengthening measures to prevent and control MDR nosocomial infections
• Monitoring MDR nosocomial infections
• Rational use of antibiotics
• National model formulary
• National essential drug lists containing 205 and 317 pharmaceuticals,
respectively
• Empiric therapy for infectious diseases
• Target therapy for microbial infections
• Pharmacological characteristics and safety of antibiotics
• Special points for antimicrobial therapy of pediatric infections
• The national monitoring network for antimicrobial use in hospitals
(mainly in tertiary & secondary hospitals)
• The national monitoring network for antibiotic resistance in hospitals
(mainly in tertiary & secondary hospitals)
• There are more than 50 training sites, and more than 2,000 pharmacists have
been trained to improve competency